A cutting-edge immunotherapy treatment will soon be offered at Rambam Health Care Campus using genetically engineered T-cells to kill cancer cells. Rambam will be the first and only medical center in Northern Israel to provide this service.
 Top Right: Professor Zukerman, courtesy of Rambam Spokesperson’s Office. Diagram: From free resource of the American Society for Transplantation and Cellular Therapy
Top Right: Professor Zukerman, courtesy of Rambam Spokesperson’s Office. Diagram: From free resource of the American Society for Transplantation and Cellular Therapy
Rambam Health Care Campus’s Department of Hematology and Bone Marrow Transplantation is launching a new service to treat several different types of cancer using altered T cells. The service will be headed by the department’s director, Professor Tsila Zuckerman.
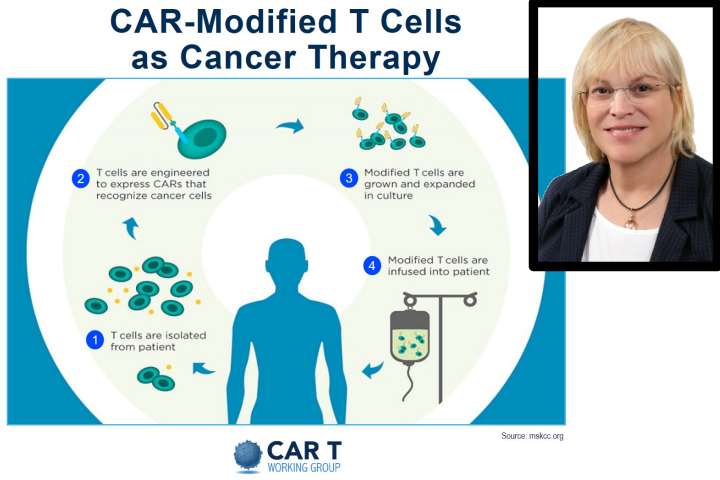
T-cells are a type of immune cell whose job is to eliminate infections and cancer cells. In order to survive, cancer cells suppress T-cell activity, enabling them to bypass the immune system. To overcome this obstacle, these cells need to undergo the process of "re-education," in which T-cells acquire the specific ability to kill tumor cells. Currently, this form of immunotherapy is primarily used to treat blood cancers like acute lymphoblastic leukemia and other advanced lymphomas, usually in cases where all other therapies (including stem cell transplantation) have failed. This innovative approach has given rise to multiple clinical trials, yielding extremely promising results.
In the procedure, a small number of immune system cells are extracted from the patient's blood, genetically reprogrammed in a special pharmaceutical lab, and then returned to the patient in a one-time treatment designed to destroy the cancer cells. This strategy entails inserting a chimeric antigen receptor (CAR)—a type of synthetic molecule designed to bind certain proteins—into the T-cells.
This treatment has been authorized by international regulatory agencies such as the FDA and EMA, as well as by the Israel Ministry of Health. Approval to provide this revolutionary treatment must undergo a rigorous accreditation process involving the Ministry of Health and the pharmaceutical companies that perform the cellular reprogramming—Novartis and Kite-Gilead. While it will be several months before approval is given, the process is on-target.
Currently, new therapeutic agents based on this approach are under intensive investigation, attempting to target other diseases such as multiple myeloma and acute myeloid leukemia. Zuckerman’s department actively treats hundreds of lymphoma, leukemia, and myeloma patients annually.
“One of the main missions in our department is to provide patients with direct access to novel therapeutic options; we aim to employ the cutting-edge CAR-T technology at our center in the very near future. To that end, we have implemented all the prerequisites required by both national and international regulatory bodies to facilitate provision of this procedure,” explains Department Director Zuckerman. Future plans include creating a dedicated immunotherapy institute.

Diagrams, from free resource of the American Society for Transplantation and Cellular Therapy

